Deep within the human circulatory system, a quiet hero has been hiding in plain sight. For decades, scientists recognized albumin as the most abundant protein in our blood, a tireless worker produced by the liver to maintain fluid balance and transport hormones. However, a groundbreaking study published in the journal Nature has revealed that this common protein holds a much more dramatic secret: it is a primary defender against one of the most terrifying pathogens known to medicine.
This adversary is mucormycosis, often referred to as “black fungus.” It is a rare but devastating infection that behaves less like a typical germ and more like an invasive, predatory weed. Once it enters the body, it grows aggressively into blood vessels, strangling the flow of life and killing the very tissue it touches. For those diagnosed, the stakes are harrowing, as the mortality rate for this fungal invader exceeds 50%. Historically, it has been a shadow that stalks the most vulnerable—those struggling with diabetes or weakened immune systems—leaving doctors to wonder why some bodies fall prey to its growth while others remain untouched.
The Mystery of the Missing Protector
The quest to understand this vulnerability led researchers to look past the fungus itself and toward the internal environment of the patients it claimed. By analyzing clinical data from hundreds of individuals, the team began to notice a startling pattern. It wasn’t just the presence of diabetes that predicted a poor outcome; it was a specific deficiency in the blood. Almost every patient who succumbed to the infection shared a common trait: dangerously low levels of albumin, a condition known as hypoalbuminemia.
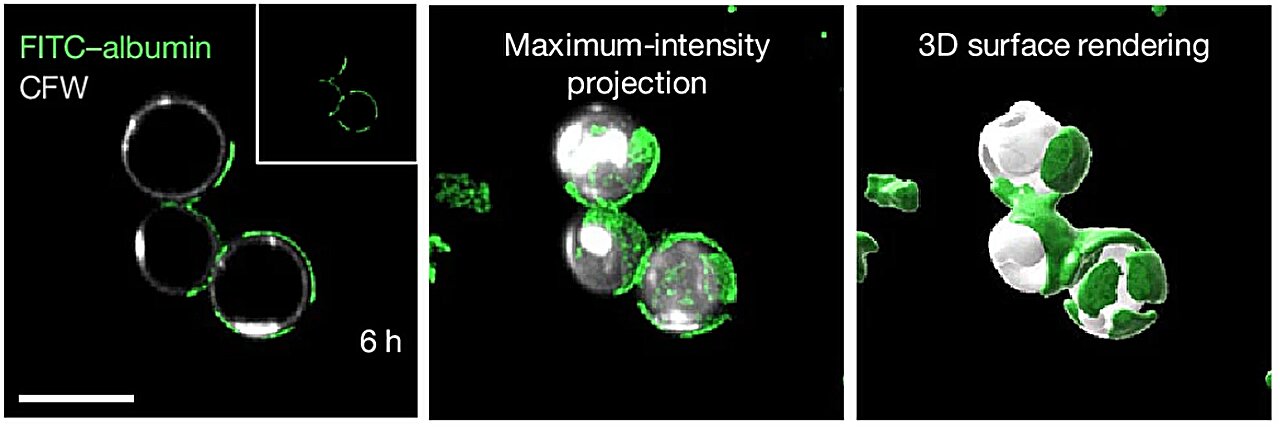
While previous medical literature had linked low protein levels to poor survival rates in various other illnesses, the specific connection to this fungal pathogen remained a mystery. The researchers hypothesized that albumin might be doing more than just carrying cargo through the veins; it might be actively policing the blood. To test this, they took healthy blood samples and performed a delicate extraction, removing the albumin entirely.
The results were immediate and chilling. Without the protein present, the fungus grew out of control, spreading with unchecked ferocity. Yet, the moment the scientists restored the albumin to the samples, the pathogen was stopped in its tracks. This marked the first time in scientific history that this specific protein was shown to have a direct, functional role in defending the human body against a fungal invader.
A Chemical Weapon Concealed in a Pocket
Determining that the protein worked was only half the battle; the scientists needed to know how a simple transport molecule could disarm a lethal killer. Upon closer inspection of the protein’s structure, they found the answer tucked away in specialized pockets designed to bind fatty acids. These pockets are not just storage containers; they are the delivery mechanism for a sophisticated chemical defense.
When albumin encounters the fungus, it undergoes a transformation. It releases these protective fatty acids, which act as a molecular shield. The target of this shield is mucoricin, a potent toxin produced by the fungus that is responsible for killing human tissue. By deploying these fats, the protein effectively “muffles” the fungus, preventing it from producing its lethal toxin. Without the ability to secrete mucoricin, the fungus loses its primary weapon, allowing the body to maintain its integrity against the invasion.
This discovery reveals that albumin acts as a master regulator of host defense. It is not merely a passive observer in the blood but an active participant that monitors for the presence of Mucorales—the order of fungi responsible for these infections—and shuts down their ability to cause harm before they can take root in the host’s tissues.
A Simple Solution for a Deadly Threat
The implications of this discovery offer a surge of hope for modern medicine, providing a roadmap for new ways to detect and treat a disease that has long been considered a death sentence. Because the link between the protein and the pathogen is so direct, the research suggests that something as simple as a blood test could save lives. By monitoring albumin levels, doctors could create an early warning system, identifying high-risk patients before the fungus ever has a chance to strike.
In the clinical setting, this knowledge could revolutionize how we approach the “black fungus.” Beyond the development of complex new drugs, the study suggests a remarkably straightforward intervention: restoring the body’s natural defenses. For patients facing a high risk of infection, providing albumin supplements to correct a deficiency could be an effective and practicable strategy to prevent the fungus from ever gaining a foothold.
Why This Research Matters
This study is a milestone in our understanding of the human immune system, proving that our most basic biological components often serve double duties as sophisticated guardians. By identifying the specific mechanism—the release of fatty acids to neutralize the mucoricin toxin—scientists have turned a mysterious, lethal threat into a manageable medical challenge.
For the thousands of people worldwide living with metabolic issues or compromised immunity, this research represents a shift from desperation to strategy. It moves the medical community away from broad-spectrum treatments and toward a targeted, elegant solution: using the body’s own most common protein to disarm its most uncommon and deadly enemy. In the fight against mucormycosis, the key to survival was never a secret medicine; it was already flowing through our veins, waiting for us to understand its power.
Study Details
Antonis Pikoulas et al, Albumin orchestrates a natural host defence mechanism against mucormycosis, Nature (2026). DOI: 10.1038/s41586-025-09882-3