When a house catches fire, the shrill scream of a smoke alarm serves one undeniable purpose: to warn everyone inside of the looming danger. We view these devices as passive sentinels, capable of shouting for help but unable to pick up a hose. However, a groundbreaking discovery by a team led by researchers at Johns Hopkins Medicine suggests that our bodies possess a biological alarm system that is far more versatile. In a study published in Science, researchers revealed that the very nerves responsible for the agonizing “scream” of a broken bone are the same ones that pick up the tools to rebuild it.
The Dual Nature of the Body’s Pain Alarm
For decades, the medical community viewed sensory neurons—the specialized nerve cells that permeate our tissues—primarily as messengers of misfortune. When a bone snaps, these peripheral afferent neurons spring into action, sending urgent electrical signals from the site of the trauma toward the central nervous system, specifically the brain and spinal column. This is the biological equivalent of an emergency call. Yet, the researchers discovered that these nerves do not just report the trauma; they undergo a radical transformation.
Once the initial alarm is sounded, these neurons morph into what the researchers call “reconstruction commanders.” Instead of merely broadcasting pain, they begin to actively direct the cellular workforce required to mend the skeletal breach. This dual function—acting as both the sensor of damage and the architect of repair—marks a shift in how we understand the relationship between our nervous system and our skeleton.
Tracing the Hidden Circuitry of Healing
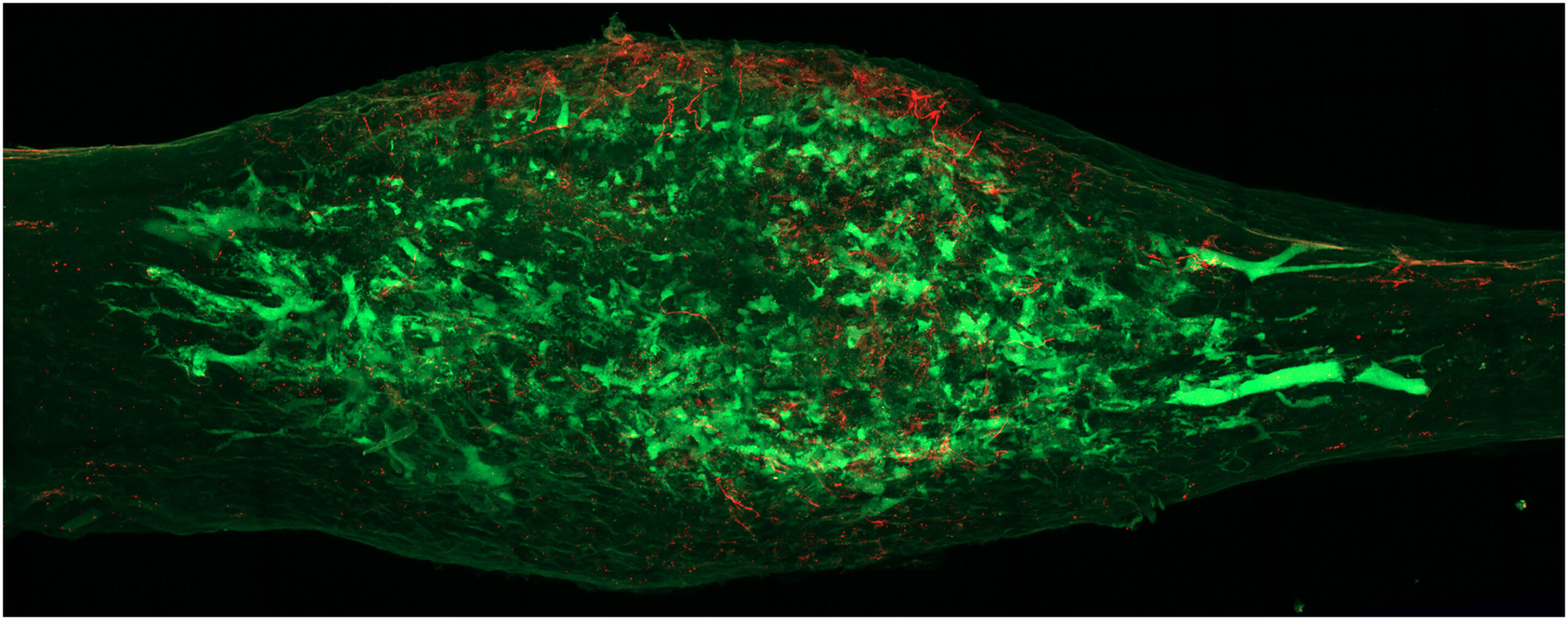
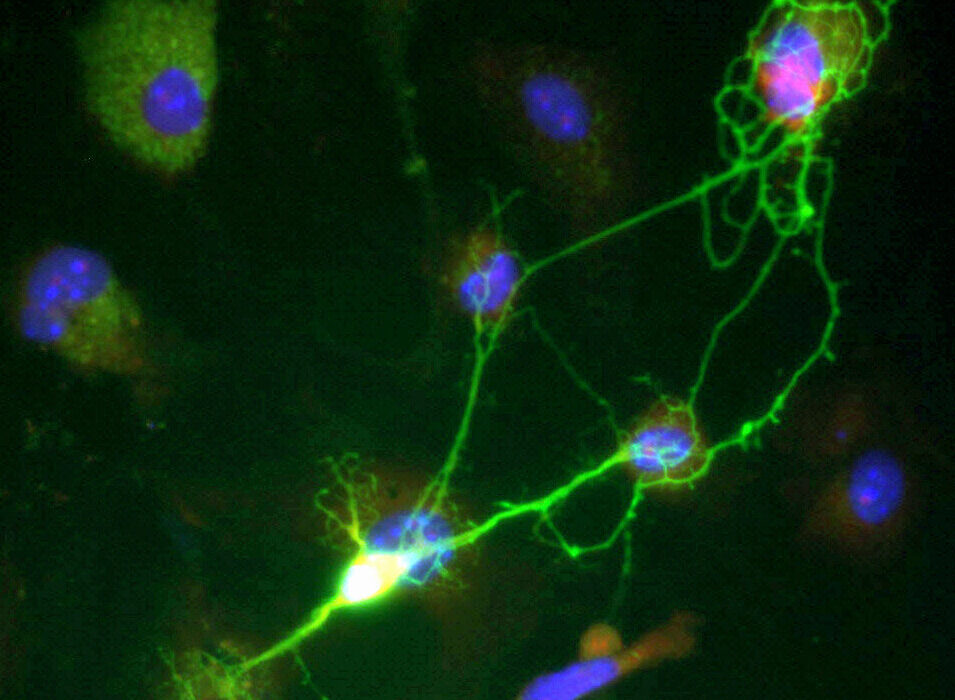
To understand how a nerve cell could possibly direct the complex process of bone formation, the team had to find a way to see the invisible wires connecting the spine to the skeleton. They utilized a laboratory-engineered adeno-associated virus, designed with a specific attraction, or tropism, for the peripheral nerves that supply the bone.
This process, known as retrograde tracing, allowed the scientists to follow the biological “wiring” in reverse. It is much like tracing a single electrical wire from a glowing light bulb back through the drywall of a house to find the exact circuit breaker that controls it. By following these viral markers, they identified the specific dorsal root ganglion (DRG) neurons—clusters of nerve cells along the spinal cord—that are dedicated to the bone.
The researchers didn’t stop at mapping the paths. They combined this tracing with single-cell RNA sequencing isolation, a high-resolution technique that allowed them to peek inside individual nerve cells before and after a fracture. By doing so, they created the world’s first comprehensive single-cell atlas of bone-innervating sensory neurons. This map provided a window into the specific proteins these cells produce, revealing the secret language the nervous system uses to talk to the skeleton.
The Molecular Keys to Reconstruction
The foundation for this discovery was laid in 2019, when the laboratory identified two critical players in the early stages of injury: nerve growth factor (NGF) and its receptor, tropomyosin receptor kinase-A (TrkA). First discovered in the 1950s, NGF is a well-known protein that helps nerves grow and maintain themselves while also alerting the brain to pain. When a bone breaks, NGF and TrkA bind together like a key in a lock, initiating a surge in nerve growth at the fracture site.
In the current study, the researchers tested what happens when this “lock” is jammed. When they genetically or chemically blocked the response of TrkA+ neurons, the results were dramatic. Not only did the nerve supply fail to grow, but the entire recovery process stalled. There was a significant reduction in blood vessel formation, a lack of bone-synthesizing cells, and a failure in the mineralization of new bone. This proved that successful fracture repair is not an autonomous process performed by the bone alone; it is strictly dependent on the signaling instructions sent by the nerves.
From Pain Messenger to Master Builder
The most fascinating revelation of the study is the “phase-shift” these neurons undergo. Immediately following an injury, the DRG neurons operate as nociceptors—specialized cells focused entirely on pain perception and triggering inflammatory responses. This is the “alarm” phase. However, as time passes, these same cells enter a pro-regenerative state.
During this second phase, the neurons begin to produce and release morphogens, signaling proteins that guide the organization and formation of living tissue. The researchers pinpointed three specific proteins of interest: transforming growth factor beta 1 (TGFB1), fibroblast growth factor 9 (FGF9), and sonic hedgehog (SHH). These proteins act as specific instructions for the body to start generating new neurons, blood vessels, and the bone and cartilage necessary to bridge the gap in a fracture.
To prove the necessity of these signals, the team performed denervation—the removal or blocking of these specific neurons—in mice with fractured bones. Without the “commands” from these nerves, the skeletal cells failed to proliferate, and stem cells could not properly differentiate into bone-building units. Through extensive analysis, the researchers singled out neuron-derived FGF9 as the essential paracrine signal—the primary cell-to-cell communication tool that makes bone repair possible.
Why This Biological Paradox Matters
This research resolves a long-standing biological paradox: why would the body send nerves specialized for sensing severe pain into the heart of a broken bone? We now know that these “pain fibers” are actually the primary drivers of bone regeneration. By bridging the fields of neuroscience, skeletal biology, and regenerative medicine, this study has identified a specific target for future medical interventions.
Identifying FGF9 as the key signal for skeletal repair opens the door for new drugs that could potentially “turn on” or enhance the healing process. This is particularly vital for patients who suffer from compromised healing, such as those with diabetes, neuropathy, or the elderly, whose natural “alarm systems” may no longer be able to transition effectively into the “reconstruction” phase. By understanding the language of the nerves, we may one day be able to whisper the instructions for healing directly to the bone, ensuring that even the most fragile patients can rebuild what was broken.
Study Details
Mingxin Xu et al, Mapping somatosensory afferent circuitry to bone identifies neurotrophic signals required for fracture healing, Science (2026). DOI: 10.1126/science.adr9608