Deep within the winding corridors of the human digestive system, a silent and constant balancing act determines the comfort of millions of Americans every single day. At the center of this biological drama is a single, fundamental question: how much water is currently flowing into the gut? When the faucet is turned too low, the result is the slow, painful grind of constipation. When the faucet is left wide open, the body faces the urgent distress of diarrhea. Despite how common these gastrointestinal struggles are, the molecular “hand” that actually grips and turns that faucet has remained a mystery for decades.
A Sixty Year Secret Hidden in Plain Sight
For over six decades, doctors have reached for a reliable tool to help patients find relief: a medication called bisacodyl. It is one of the most widely used laxatives in the world, trusted for its ability to soften stools and restart the digestive rhythm. Yet, surprisingly, the scientific community did not actually know how it worked. We knew that taking the pill led to a specific result, but the exact mechanical “click” it made inside the human body was invisible.
A team of researchers from Northwestern University, in collaboration with China Pharmaceutical University, decided to hunt down this missing link. They weren’t just looking for a drug target; they were searching for the master regulator of intestinal fluid. Their journey took them from the broad observations of animal physiology down to the infinitesimal world of atomic-level interactions. What they found, recently published in the journal Nature Communications, has finally solved a long-standing medical riddle and revealed a hidden switch that governs our internal hydration.
The Discovery of the Master Molecular Switch
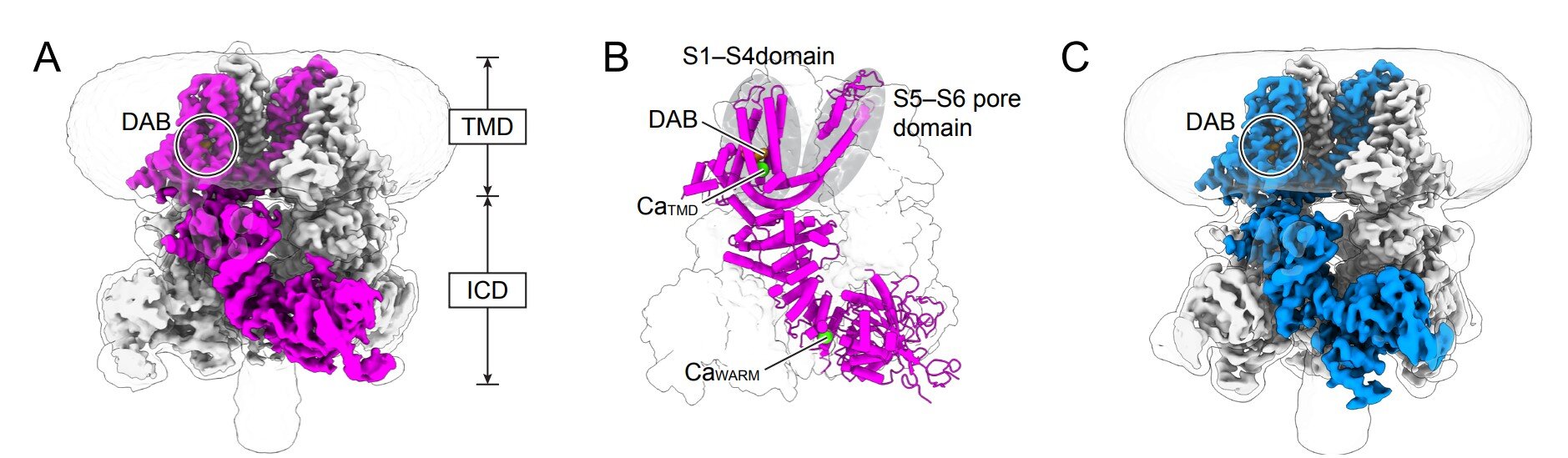
The breakthrough centered on a specific protein known as an ion channel called TRPM4. While the name might sound like technical jargon, its role is essentially that of a gatekeeper. This channel sits within the epithelial cells that line the walls of our intestines. These cells act as the ultimate border patrol, deciding exactly how much salt and water are allowed to move from the body’s tissues into the gut.
The researchers discovered that TRPM4 acts as the “master switch” for this entire process. When this switch is flipped, it triggers a sophisticated domino effect. First, it allows sodium ions to rush into the epithelial cells. This sudden influx of sodium creates an electrical shift that pulls calcium into the cell. This secondary surge then activates a chloride channel, which releases chloride ions out into the gut. Because water naturally follows salt, a wave of fluid pours into the intestine. This is the biological “faucet” being turned on, providing the lubrication and volume necessary for a laxative effect.
Peering into a Hidden Pocket at the Atomic Scale
To understand how a simple pill could trigger such a complex chain reaction, the team utilized a high-tech imaging method called cryo-electron microscopy. This allowed them to visualize the TRPM4 channel at a resolution so high they could see individual atoms. It was during this deep dive into the protein’s architecture that they found something no one had ever seen before: a previously unknown drug-binding pocket.
In the past, scientists believed that TRPM4 only responded to calcium signals already present inside the cell. However, the researchers found that the active form of the drug, deacetyl bisacodyl, doesn’t wait for a calcium signal. Instead, it tucks itself perfectly into this hidden pocket like a key into a lock. Once seated there, it forces the channel into an active state, bypassing the usual cellular requirements. This discovery was the “smoking gun” that explained how bisacodyl exerts such a powerful influence on the gut.
To prove that TRPM4 was indeed the primary player, the researchers conducted a definitive test using mouse models. In healthy mice, the drug worked perfectly to increase water content and soften stools. However, in mice that had been genetically engineered to lack the TRPM4 channel, the drug was essentially a ghost—it had no effect whatsoever. This confirmed that without this specific molecular gate, the body’s primary mechanism for chemical-induced fluid release simply does not function.
The Warmth of a Living System
This discovery was not an overnight success but the culmination of years of intense focus. Back in 2017, the labs at Northwestern published the first atomic-resolution structures of TRPM4, which gave the world a glimpse of how the channel is assembled. But a protein in a laboratory dish doesn’t always behave like a protein in a living body.
In 2024, the researchers made another vital observation: the shape of the TRPM4 channel changes based on physiological temperature. When the channel is “warm”—at the temperature of a living human body—it adopts a specific conformation that is essential for its ability to open and close. This context was critical for the recent study, as it allowed the scientists to see how the drug binds to the channel in a real-world, biological environment. It turns out that temperature profoundly reshapes how the drug and the channel interact, providing a roadmap for how these systems operate in a living, breathing organism.
Why This Molecular Roadmap Matters for Everyone
The identification of the TRPM4 signaling axis is far more than just a win for scientific curiosity; it is a blueprint for the future of gastrointestinal medicine. By defining this “water faucet” at the atomic level, researchers have opened the door to a new generation of targeted treatments that could be much more precise than the broad-spectrum medications used today.
On one side of the coin, scientists can now look for new molecules that activate this channel even more efficiently to treat chronic constipation, helping millions of people who find no relief from current options. On the other side, and perhaps even more significantly, this discovery offers a path to treating the opposite problem. If TRPM4 is the switch that turns the water on, then designing a drug to inhibit or “block” this channel could effectively turn the water off. This would provide a brand-new way to curb diarrhea, a condition that remains a major health challenge globally.
By understanding this newly defined signaling axis, doctors and researchers finally have a clear view of how the gut maintains its delicate fluid balance in health—and exactly what to do when that balance is disrupted by disease. It is a rare, comprehensive look at a drug’s journey, starting from a single atom and ending with the comfort and health of the entire human body.
Study Details
Yaru Liu et al, Noncanonical calcium-independent TRPM4 activation governs intestinal fluid homeostasis, Nature Communications (2026). DOI: 10.1038/s41467-025-68014-7