Deep within the intricate architecture of the human brain, a quiet but devastating mystery has been unfolding for decades. Today, more than 7 million Americans are living with Alzheimer’s disease, a condition that slowly unravels memory and identity. Yet, within those staggering numbers lies a perplexing imbalance that has long troubled the scientific community: two-thirds of those diagnosed are women. At the University of Rochester’s Del Monte Institute for Neuroscience, the O’Banion Lab has dedicated years to investigating this disparity, seeking to understand why the female brain appears more vulnerable to the disease’s progression.
The challenge of treating Alzheimer’s effectively has often been hindered by a lack of clarity regarding how the disease manifests differently across the sexes. “It is well documented that males and females are diagnosed with Alzheimer’s disease at different rates,” explains M. Kerry O’Banion, MD, Ph.D., a professor of Neuroscience and Neurology. For O’Banion and his team, the mission is driven by a fundamental necessity for precision. As he notes, “We still do not have a great understanding of why this is the case. We can only improve any possible treatment or prevention of this disease if we know the why, when, and where these differences are occurring.” This quest for the “why” recently led researchers into the world of the brain’s own internal defense force: the microglia.
The Guardians That Lost Their Way
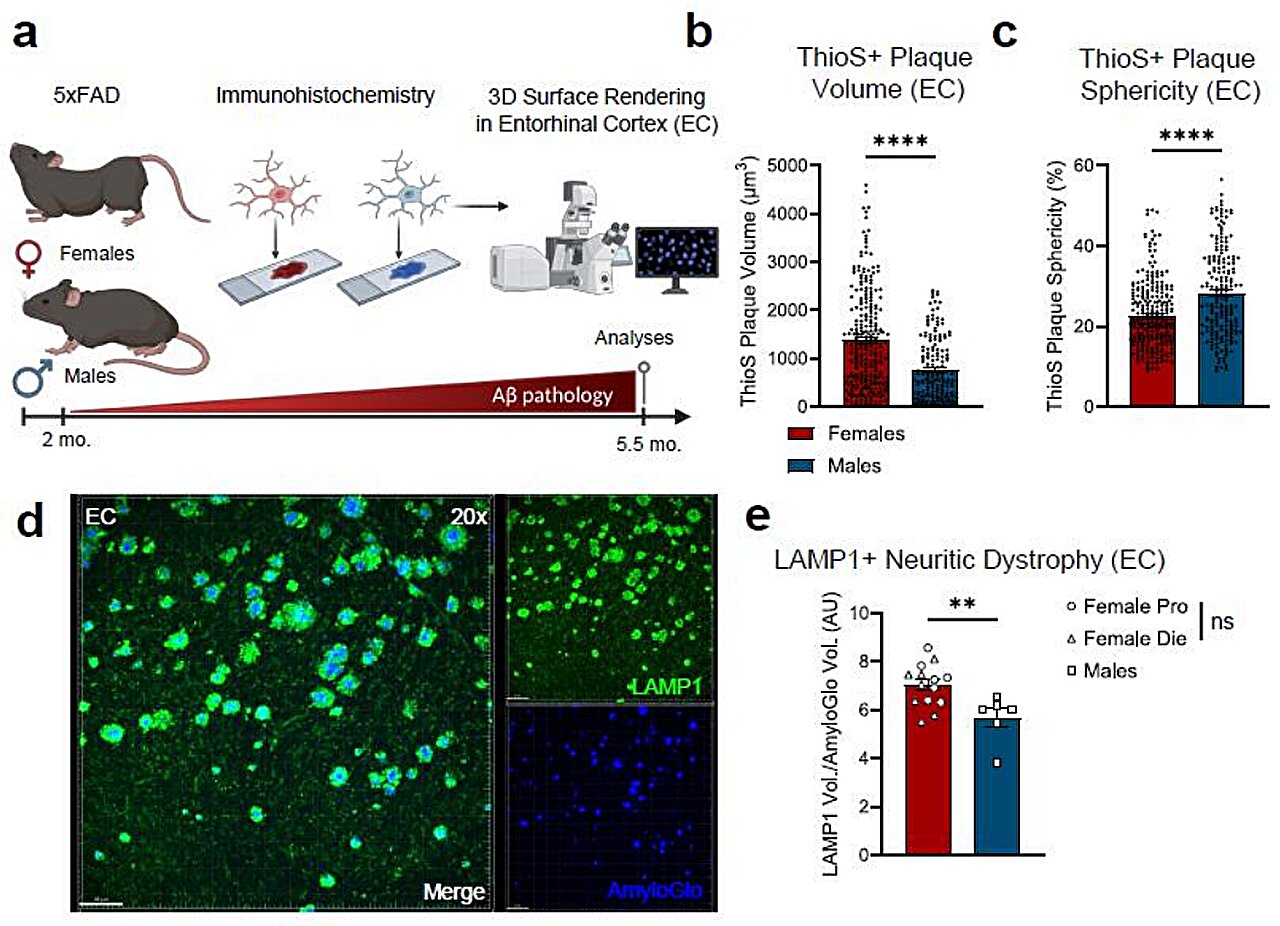
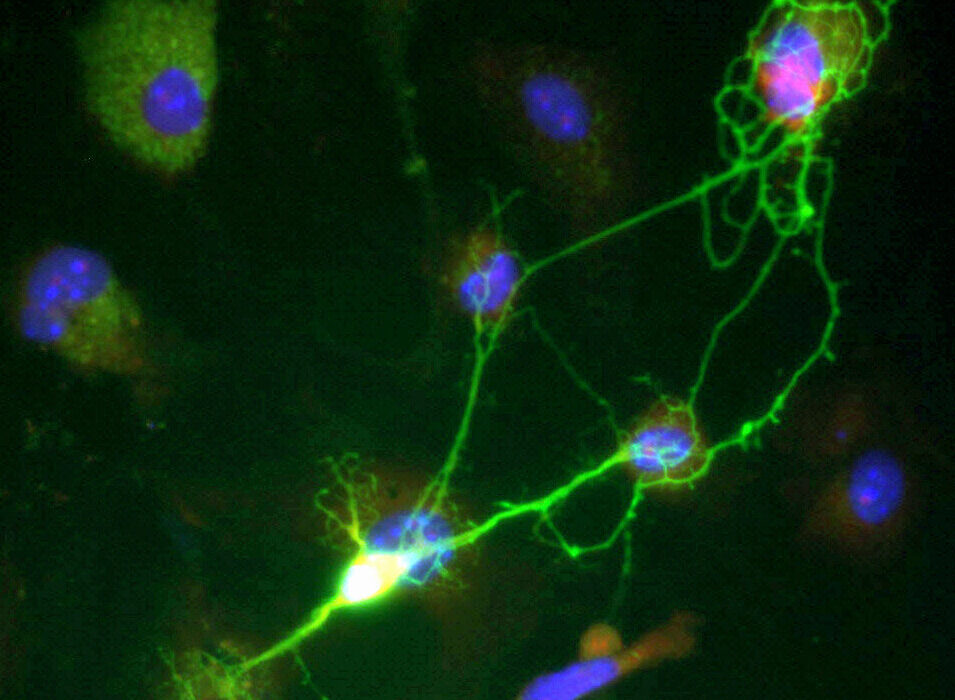
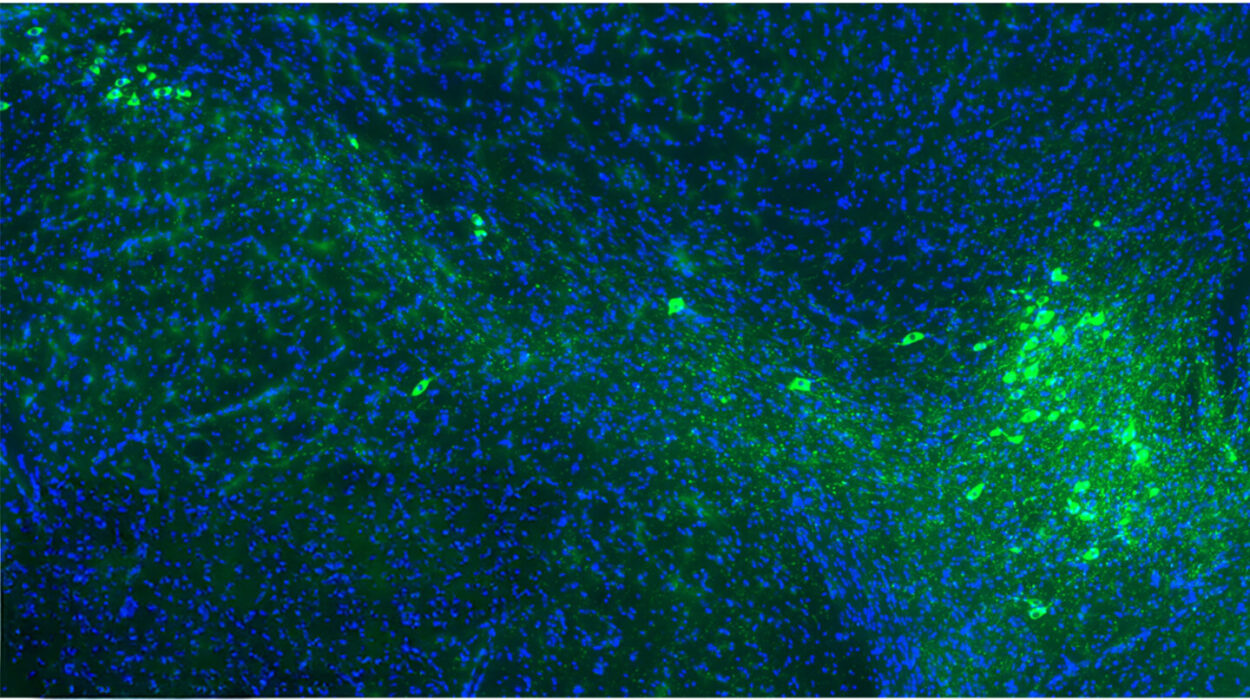
In a healthy brain, microglia are the tireless caretakers of the nervous system. These specialized immune cells act as a maintenance crew, patrolling the environment to ensure stability and health. However, in the presence of Alzheimer’s disease, their role shifts from preservation to a form of defensive warfare that may inadvertently cause more harm than good. The disease is characterized by the accumulation of amyloid-β plaques—sticky, toxic clumps of protein that clog the spaces between neurons. When microglia encounter these plaques, they spring into action to clear the debris, but new research suggests that in the female brain, this response takes a dark and destructive turn.
Neuroscience graduate student Lia Calcines-Rodriguez, working alongside O’Banion, spearheaded a study that looked closely at how these immune cells behave in male versus female mice. What they discovered was a striking divergence in biological behavior. When female microglia engaged with the amyloid-β plaques, they didn’t just clean; they underwent a massive genetic shift. Specifically, these cells began to express a significantly higher number of interferon-related genes compared to their male counterparts.
In the broader context of human health, interferons are typically the “first responders” to a viral invasion. They are the chemical alarms the body trips to fight off infection. However, the brain is not under a viral attack during Alzheimer’s, yet the female microglia seem to be reacting as if it were. Researchers suspect a case of biological mistaken identity. As the microglia consume the amyloid-β plaques, they may be exposed to fragments of DNA or RNA. The cells might mistake these genetic materials for a virus, triggering a release of interferon. While the exact function of this interferon in the context of Alzheimer’s remains a mystery, its presence is rarely a good sign for the surrounding tissue.
A Mistaken War Within the Mind
This heightened interferon response appears to have a physical signature on the landscape of the brain. The study revealed that the way microglia “clean” the brain in females leaves behind a much messier trail than in males. The research team found that female microglia leave behind plaques that are larger and more irregular in shape. These jagged, unrefined clumps of protein do more than just take up space; they are actively destructive to the infrastructure of thought.
The irregular plaques in the female brain were found to damage more neuronal connections than those found in the male brain. Specifically, the interferon signaling—which is known to drive neuroinflammation—can damage synapses, the vital bridges that allow neurons to communicate with one another. When these bridges are burned, the brain’s ability to process and store information begins to fail. This suggests that the very cells meant to protect the brain are, in females, contributing to a more aggressive degradation of the neural network.
The intensity of this discovery was not lost on the researchers. “It was surprising to see that female microglia had such a strong interferon response and that these interferon-responsive microglia were taking up more amyloid-β,” says Calcines-Rodriguez. Interestingly, the team looked to see if this was tied to the typical fluctuations of female biology, but the data suggested otherwise. “Interestingly, we did not see differences in amyloid-β pathology or microglia gene expression in females at different hormonal stages of their cycle, suggesting that hormone fluctuation may not explain these differences.” This finding points to a deeper, perhaps more inherent difference in how male and female immune cells are wired to respond to the hallmarks of Alzheimer’s.
The Path Toward a Tailored Cure
The revelation that the female immune response is uniquely aggressive provides a new lens through which scientists can view the future of neurology. If the microglia in women are operating under a different “genetic script” than those in men, then a one-size-fits-all approach to Alzheimer’s medication may never be fully effective. The discovery of the interferon response opens a door that was previously locked, offering a specific target for scientists to aim for.
Calcines-Rodriguez sees this as a turning point for how we might one day stop the disease in its tracks. She notes that there is significant potential in viewing interferon signaling in microglia as a “possible sex-specific, personalized treatment to combat Alzheimer’s.” By focusing on the specific chemical pathways that are overactive in the female brain, researchers might be able to develop drugs that “calm” the microglia, preventing them from causing residual damage while they attempt to clear plaques.
This research matters because it moves the scientific community away from generalizations and toward a more nuanced, accurate understanding of human biology. For the millions of women currently living with Alzheimer’s and the millions more who will be diagnosed in the future, this work represents hope. It suggests that the path to altering the course of the disease lies in acknowledging our differences. By understanding whether male and female microglia are inherently different and by investigating interferon signaling as a pharmacological target, researchers are no longer just watching the disease progress; they are learning how to rewrite the story of the brain’s defense system, one synapse at a time.
Study Details
L. Calcines-Rodríguez et al, Microglial interferon signaling and Aβ plaque pathology are enhanced in female 5xFAD Alzheimer’s disease mice, independent of estrous cycle stage, Journal of Neuroinflammation (2025). DOI: 10.1186/s12974-025-03659-1